Who is Emergent Travel Health?
Emergent is committed to improving travel health–not just by the products we sell, but also by the resources and tools we provide
Emergent is committed to improving travel health–not just by the products we sell, but also by the resources and tools we provide

Emergent Travel Health is a group within Emergent BioSolutions. We currently supply VIVOTIF® (Typhoid Vaccine Live Oral Ty21a) and VAXCHORA® (Cholera Vaccine, Live, Oral), and have innovative solutions in our pipeline.
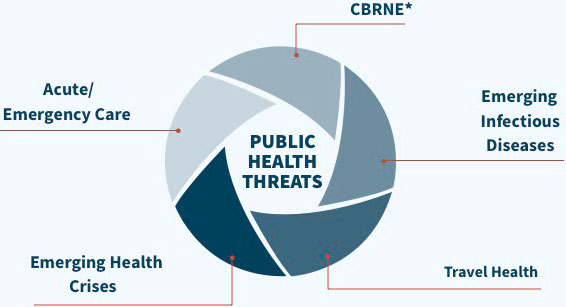
Emergent BioSolutions is a global life sciences company with a diversified portfolio of products focused on addressing threats to public health.
The goal of Emergent BioSolutions is to enhance and protect 1 billion lives by 2030 and bring peace of mind to an uncertain world.
Our company is built on the foundation of an inclusive, values-driven culture; one with high employee engagement and where a diversity of experiences, backgrounds, and skills drive innovation and operational excellence.

VIVOTIF is indicated for immunization of adults and children greater than 6 years of age against disease caused by Salmonella enterica serovar Typhi.
Routine typhoid vaccination is not recommended in the United States of America. Selective immunization against typhoid fever is recommended for the following groups: 1) travelers to areas in which there is a recognized risk of exposure to S. Typhi; 2) persons with intimate exposure (e.g., household contact) to an S. Typhi carrier; and 3) microbiology laboratorians who work frequently with S. Typhi. There is no evidence to support the use of typhoid vaccine to control common source outbreaks, disease following natural disasters, or in persons attending rural summer camps.
Not all recipients of VIVOTIF will be fully protected against typhoid fever. Vaccinated individuals should continue to take personal precautions against exposure to typhoid organisms. The vaccine will not afford protection against species of Salmonella other than Salmonella Typhi or other bacteria that cause enteric disease. The vaccine is not suitable for treatment of acute infections with S. Typhi.
VIVOTIF is contraindicated in patients with a hypersensitivity to any component of the vaccine or the enteric-coated capsule. The vaccine should not be administered to persons during an acute febrile illness. Safety of the vaccine has not been demonstrated in persons deficient in their ability to mount a humoral or cell-mediated immune response, due to either a congenital or acquired immunodeficient state including treatment with immunosuppressive or antimitotic drugs. The vaccine should not be administered to these persons regardless of benefits.
Acute Gastrointestinal Illness: VIVOTIF is not to be taken during an acute gastrointestinal illness. Postpone taking the vaccine if persistent diarrhea or vomiting occurs.
Concomitant Administration with Sulfonamides and Antibiotics: The vaccine should not be administered to individuals receiving sulfonamides and antibiotics since these agents may be active against the vaccine strain and prevent a sufficient degree of multiplication to occur in order to induce a protective immune response.
Diminished Immune Response: Unless a complete immunization schedule is followed, an optimum immune response may not be achieved. Not all recipients of VIVOTIF will be fully protected against typhoid fever.
Personal Precautions: Vaccinated individuals should continue to take personal precautions against exposure to typhoid organisms (i.e., travelers should take all necessary precautions to avoid contact or ingestion of potentially contaminated food or water).
Concomitant Administration with Anti-malarial Drugs: Several anti-malaria drugs, such as mefloquine, chloroquine and proguanil (not approved for use in US) possess anti-bacterial activity which may interfere with the immunogenicity of VIVOTIF. A study showed that mefloquine and chloroquine can be administered together with VIVOTIF. Proguanil should be administered only if 10 days or more have elapsed since the final dose of VIVOTIF was ingested.
Adverse Reactions: The most common adverse reactions in clinical trials were abdominal pain (6.4%), nausea (5.8%), headache (4.8%), fever (3.3%), diarrhea (2.9%), vomiting (1.5%) and skin rash (1.0%). Only the incidence of nausea occurred at a statistically higher frequency in the vaccinated group as compared to the placebo group.
VIV FULL HCP ISI 07/2020

VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera‑affected areas.
Limitations of Use: The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas. The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine. VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups.
VAXCHORA is contraindicated in persons who have a history of severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine.
Altered Immunocompetence: The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons and the immunologic response to VAXCHORA may be diminished in immunocompromised individuals.
Shedding and Transmission: Because VAXCHORA may be shed in the stool of recipients for at least 7 days and the vaccine strain can potentially be transmitted to non‑vaccinated close contacts (e.g., household contacts), use caution when considering whether to administer VAXCHORA to individuals with immunocompromised close contacts.
Adverse Reactions: In adults 18-45 years old, the most common adverse reactions (incidence > 3%) were tiredness (31%), headache (29%), abdominal pain (19%), nausea/vomiting (18%), lack of appetite (17%) and diarrhea (4%).
The most common adverse reactions for children and adolescents (incidence ≥10%) were:
Antibiotics: Avoid concomitant administration of VAXCHORA with oral systemic antibiotics since these agents may be active against the vaccine strain. Do not administer VAXCHORA to patients who have received oral or parenteral antibiotics within 14 days prior to vaccination.
Antimalarial Prophylaxis: Immune responses to VAXCHORA may be diminished when administered concomitantly with chloroquine. Administer VAXCHORA at least 10 days before beginning chloroquine.
Immunosuppressive Treatments: Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids, may reduce the immune response to VAXCHORA.
VAX FULL HCP ISI 01/2021

VIVOTIF is indicated for immunization of adults and children greater than 6 years of age against disease caused by Salmonella enterica serovar Typhi.
VIVOTIF is indicated for immunization of adults and children greater than 6 years of age against disease caused by Salmonella enterica serovar Typhi.
Routine typhoid vaccination is not recommended in the United States of America. Selective immunization against typhoid fever is recommended for the following groups: 1) travelers to areas in which there is a recognized risk of exposure to S. Typhi; 2) persons with intimate exposure (e.g., household contact) to an S. Typhi carrier; and 3) microbiology laboratorians who work frequently with S. Typhi. There is no evidence to support the use of typhoid vaccine to control common source outbreaks, disease following natural disasters, or in persons attending rural summer camps.
Not all recipients of VIVOTIF will be fully protected against typhoid fever. Vaccinated individuals should continue to take personal precautions against exposure to typhoid organisms. The vaccine will not afford protection against species of Salmonella other than Salmonella Typhi or other bacteria that cause enteric disease. The vaccine is not suitable for treatment of acute infections with S. Typhi.
VIVOTIF is contraindicated in patients with a hypersensitivity to any component of the vaccine or the enteric-coated capsule. The vaccine should not be administered to persons during an acute febrile illness. Safety of the vaccine has not been demonstrated in persons deficient in their ability to mount a humoral or cell-mediated immune response, due to either a congenital or acquired immunodeficient state including treatment with immunosuppressive or antimitotic drugs. The vaccine should not be administered to these persons regardless of benefits.
Acute Gastrointestinal Illness: VIVOTIF is not to be taken during an acute gastrointestinal illness. Postpone taking the vaccine if persistent diarrhea or vomiting occurs.
Concomitant Administration with Sulfonamides and Antibiotics: The vaccine should not be administered to individuals receiving sulfonamides and antibiotics since these agents may be active against the vaccine strain and prevent a sufficient degree of multiplication to occur in order to induce a protective immune response.
Diminished Immune Response: Unless a complete immunization schedule is followed, an optimum immune response may not be achieved. Not all recipients of VIVOTIF will be fully protected against typhoid fever.
Personal Precautions: Vaccinated individuals should continue to take personal precautions against exposure to typhoid organisms (i.e., travelers should take all necessary precautions to avoid contact or ingestion of potentially contaminated food or water).
Concomitant Administration with Anti-malarial Drugs: Several anti-malaria drugs, such as mefloquine, chloroquine and proguanil (not approved for use in US) possess anti-bacterial activity which may interfere with the immunogenicity of VIVOTIF. A study showed that mefloquine and chloroquine can be administered together with VIVOTIF. Proguanil should be administered only if 10 days or more have elapsed since the final dose of VIVOTIF was ingested.
Adverse Reactions: The most common adverse reactions in clinical trials were abdominal pain (6.4%), nausea (5.8%), headache (4.8%), fever (3.3%), diarrhea (2.9%), vomiting (1.5%) and skin rash (1.0%). Only the incidence of nausea occurred at a statistically higher frequency in the vaccinated group as compared to the placebo group.
VIV FULL HCP ISI 07/2020

VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera‑affected areas.
VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera‑affected areas.
Limitations of Use: The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas. The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine. VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups.
VAXCHORA is contraindicated in persons who have a history of severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine.
Altered Immunocompetence: The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons and the immunologic response to VAXCHORA may be diminished in immunocompromised individuals.
Shedding and Transmission: Because VAXCHORA may be shed in the stool of recipients for at least 7 days and the vaccine strain can potentially be transmitted to non‑vaccinated close contacts (e.g., household contacts), use caution when considering whether to administer VAXCHORA to individuals with immunocompromised close contacts.
Adverse Reactions: In adults 18-45 years old, the most common adverse reactions (incidence > 3%) were tiredness (31%), headache (29%), abdominal pain (19%), nausea/vomiting (18%), lack of appetite (17%) and diarrhea (4%).
The most common adverse reactions for children and adolescents (incidence ≥10%) were:
Antibiotics: Avoid concomitant administration of VAXCHORA with oral systemic antibiotics since these agents may be active against the vaccine strain. Do not administer VAXCHORA to patients who have received oral or parenteral antibiotics within 14 days prior to vaccination.
Antimalarial Prophylaxis: Immune responses to VAXCHORA may be diminished when administered concomitantly with chloroquine. Administer VAXCHORA at least 10 days before beginning chloroquine.
Immunosuppressive Treatments: Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids, may reduce the immune response to VAXCHORA.
VAX FULL HCP ISI 01/2021